prodotti
servizi
formazione
negozio
contatti
azienda
Global Headquarters
2300 Riverchase Center
Birmingham, AL 35244
USA
tel: 888.246.8338
tel: 205.967.7880
fax: 205.870.0304
BioHorizons Canada
21 Amber Street, Unit #6
Markham, Ontario L3R 4Z3
Canada
tel: 866.468.8338
fax: 905.944.1894
BioHorizons Chile, S.A.
Av. Manquehue Norte 1337 Office 31
Vitacura
Santiago
Cile
tel: +56 (2) 23619519
fax: +56 (2) 361.9521
BioHorizons Italia Srl
Via Ettore Cristoni, 88
40033 Casalecchio di Reno (BO) Italia
numero verde +800.063.040
tel. +39.051.59.07.00
fax +39.051.57.61.06
BioHorizons México
Av. Insurgentes # 64 colonia Juarez
Torre B oficina 407
Delegación Cuauhtémoc
CP 06600 CDMX
Messico
tel. 800.953.0498
tel. 011.52.5511638675
Spanish Office
BioHorizons Ibérica
Calle Oruro, 9
28016 Madrid,
Spagna
tel: +34.91.713.10.84
fax: +34.91.355.83.75
BioHorizons Camlog UK & Ireland
Reflex
Cain Road
Bracknell
Berkshire RG12 1HL
Regno Unito
tel: +44 (0)1344 752560
Grafton DBM
Grafton DBM
Grafton DBM integra la potente tecnologia DBF (Demineralized Bone Fibers) per garantire una osteoconduttività superiore. Con oltre vent'anni di ricerca clinica alle spalle, Grafton DBM consente di preservare l’altezza e la larghezza dell’osso.1 Di dimostrata efficacia in studi clinici peer reviewed pubblicati, i vari formati di Grafton DBM rappresentano una soluzione nelle applicazioni di innesto osseo.2
- Indicati come riempitivi per cavità ossee, estensioni per innesti ossei e sostituti di innesti ossei.3
- Sono disponibili più formati che consentono di ottenere le caratteristiche di manipolazione desiderate per numerose indicazioni cliniche

Layout Style for Content Bar
possibili applicazioni4
- innesto alveolare post estrattivo
- aumento del volume crestale e del seno mascellare
- difetti cistici
- rialzo craniofacciale
- riempimento di difetti parodontali

casi Grafton DBM
aumento del volume crestale
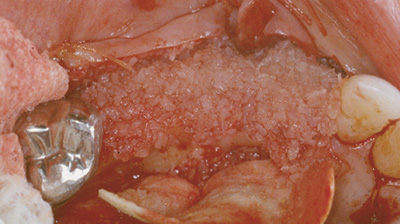
innesto composito di Grafton DBM e FDBA
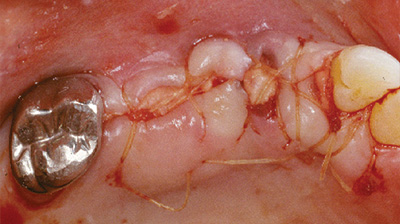
Alloderm GBR viene utilizzato come membrana ad effetto barriera
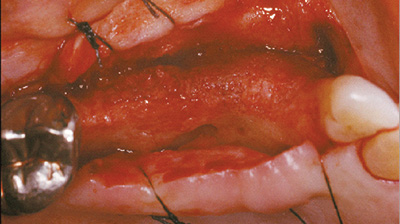
profondità rigenerata per permettere il posizionamento dell’impianto
Layout Style for Content Bar
Letteratura
Select Additional Info Type
- Histologic Analysis Of Implant Sites After Grafting With Demineralized Bone Matrix Putty And Sheets. Callan DP, Salked, SL, Scarborough, NL Implant Dent. 2009;9(1):36-42.
- I formati Grafton DBM e Grafton Plus DBM in pasta sono prodotti approvati FDA 510(k) per l'utilizzo come riempitivi per cavità ossee, estensioni per innesti ossei e sostituti di innesti ossei.
- FDA 510(k) K051188 e K051195: Indicazioni per dichiarazioni d’uso e riassunti sulla sicurezza e l’efficacia per l'utilizzo come riempitivi per cavità ossee, estensioni per innesti ossei e sostituti di innesti ossei.
- Foglietto illustrativo con Istruzioni per l'uso (IFU) del produttore.








