American Made Dental Implants
Since our beginning, BioHorizons has manufactured dental implants in the U.S.
Tapered Pro Conical
streamlined clinical performance
BioHorizons Tapered Pro Conical dental implants are based on the clinically proven, user-friendly CONELOG connection and successful BioHorizons buttress thread design.
Non disponibile in tutti i Paesi.
fueling full-arch workflows with
Tapered Pro Conical
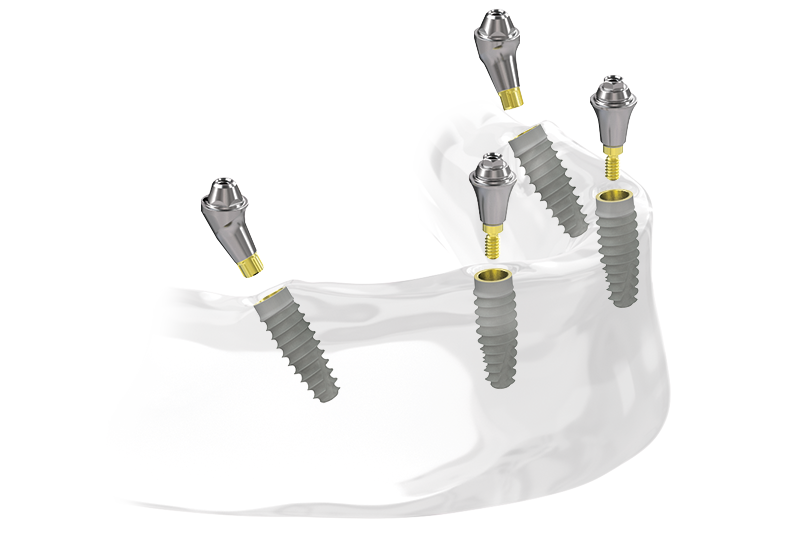
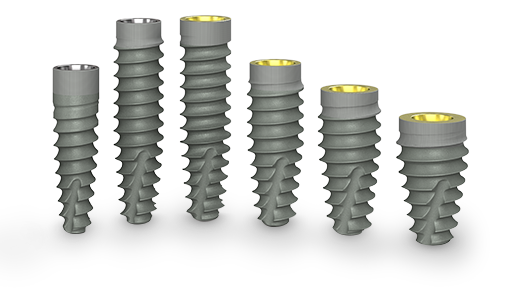
BioHorizons Tapered Pro Conical implant offers the 3.8mm diameter implant in two prosthetic platforms: narrow and regular. This expanded portfolio streamlines full-arch workflows by allowing the use of a single prosthetic platform for any full-arch case.
SmartShape™ Healers
Designed to simplify treatment workflows and deliver superior esthetic outcomes.
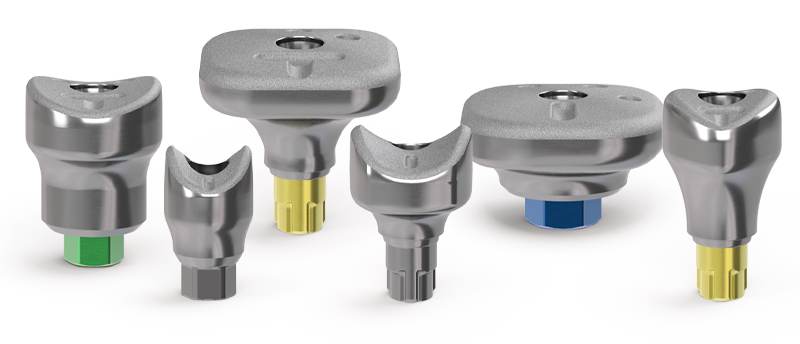
Anatomically shaped healing abutments that are integrated with digital and traditional restorative workflows.

Pro Surgical System
Keyless guided and freehand workflows in a single kit, for a new level of flexibility and predictability

Tapered Short Guided System — shorter. simpler. faster.
The BioHorizons Tapered Short Guided kit offers a new approach to guided surgery, specifically designed for the Tapered Short implants. The updated instrument designs minimize stack height while the keyless drill design simplifies the surgical workflow.
conosciamo...
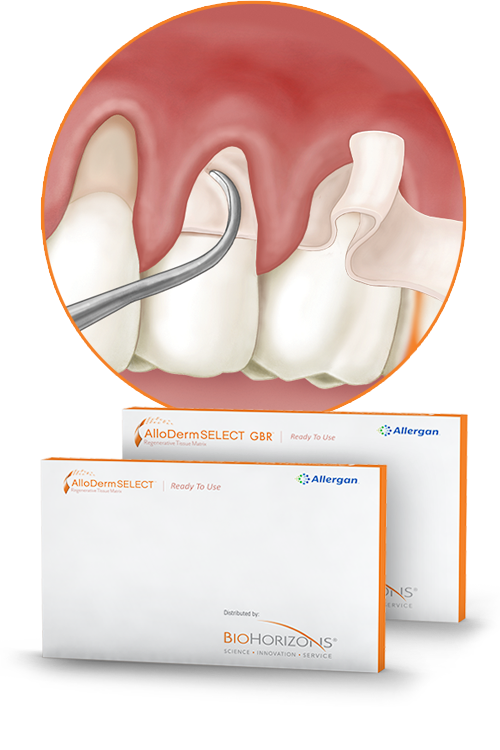
AlloDerm SELECT™ RTM
the trusted performance of AlloDerm™ RTM with additional proven benefits
Since its introduction to dentistry in 1999, AlloDerm SELECT™ Regenerative Tissue Matrix (RTM) has been a widely accepted acellular dermal matrix (ADM) for soft tissue applications. As demonstrated in preclinical studies, AlloDerm SELECT™ supports tissue regeneration by allowing rapid revascularization and cell repopulation*1,2—ultimately transitioning into host tissue for a strong repair.
- No recognizable difference between AlloDerm SELECT™ RTM and connective tissue in terms of recession reduction, clinical attachment gain, and reduction in probing depth at six months4,‡
- La matrice di tessuto rigenerativo con più studi pubblicati in implantologia5
- Sterile and ready-to-use6